By Hailian Tang
Heterogeneous catalysis plays a central role in modern chemical industry. The demand to develop new catalytic materials may be endless due to our continuous pursuit of better catalytic performance or of new and green catalytic processes. Finding the right catalytic materials, however, has many elements of trial and error and thus is usually a tedious, time-consuming process. Therefore, the catalyst development by rational design on the basic of understanding the catalytic mechanism and knowing the properties of the materials has been a long-term dream and is regarded as one of the greatest challenges.
In the past decades, oxides supported gold nanoparticles or clusters as catalysts have attracted a great deal of and yet increasing interest in catalysis field. On the one hand, supported Au catalysts have demonstrated unique catalytic performance for numerous important chemical reactions. On the other hand, the performances of supported Au catalysts were dramatically influenced by many factors. The latter is not only fundamentally interesting but also practically important because an in-depth understanding of why and how these factors affected the catalyst could be helpful to modify the catalyst formula thus promoting the catalyst performance. Of more importance, the learning gained from the studies of supported Au catalysts may provide reference or guidance to other noble metal catalyst design and development.
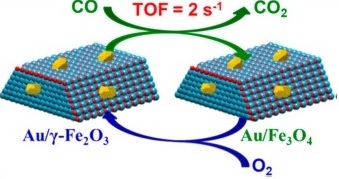
A previous research (Journal of catalysis, 2013, 299, 90-100) by the Laboratory of Catalysts and New Materials for Aerospace demonstrated that the CO oxidation on the Au/ferrihydrite catalyst was mainly according to a redox mechanism, i.e., the ferrihydrite support with high redox property led to a high activity of the catalyst. Maghemite (γ-Fe2O3) has the reverse spinel structure similar to magnetite (Fe3O4), and the reduction of γ-Fe2O3 is just related the losing of O but does not involve any lattice rearrangement. Therefor the reduction of γ-Fe2O3 at low temperature is very likely to be realized with the assistant of gold nanoparticles/clusters. With this consideration, we by using the commercial γ-Fe2O3 as support developed an exceptionally active Au/γ-Fe2O3 catalyst. The Au/γ-Fe2O3 catalyst exhibits a catalytic activity about 20 times higher than that of the Au/α-Fe2O3 catalyst for CO oxidation and presents one of the most active supported gold catalysts ever reported, showing the significant effect of support crystal phase. Systematic study shows that this support crystal phase effect could be extended to γ-Fe2O3 supported Pt-group metals and to other reactions that follow Mar-Van Krevelen (redox) mechanism. This finding may provide a new avenue for catalyst improvement or development by choosing the suitable crystal phase of the oxide support. This work has been recently published in ACS catalysis (ACS Catalysis,2015, 5, 3528-3539).