Recently, our Center used the self-developed in-situ/operando electrochemical Mössbauer spectroscopy device to conduct an in-depth exploration of the mechanism of Ni-Fe based catalysts in the electrocatalytic oxygen evolution reaction (OER). A large amount of Fe4+ was first observed near the onset potential of OER through experiments, and it was further confirmed that the current density of OER is positively associated with the content of high-valent iron species which deepened people’s understanding the reaction mechanism of Fe sites of Ni-Fe based catalysts in the OER reaction.
The research of electrocatalytic oxygen evolution materials is of great significance to the development of hydrogen energy and metal-air batteries. However, most of catalyst for commercialization are Ru, Ir-based and other noble metal catalysts. The development of non-precious metal OER catalysts with excellent performance and low price has been a hot research topic in recent years. At the same time, using in-situ/operando Mössbauer spectroscopy and other methods to study the reaction mechanism of OER materials to guide the synthesis of high-performance OER catalyst materials has become an important means to explore OER materials. For Ni-Fe oxyhydroxide OER catalysts, in-situ/operando Mössbauer spectroscopy studies have been reported, and it is found that there are indeed high-valent iron species in the reaction, but it is observed that it is generated at a relatively higher potential, which is much higher than OER. It is also reported that the kinetics can’t effectively promote the OER reaction. (JACS, https://pubs.acs.org/doi/10.1021/jacs.5b10699).
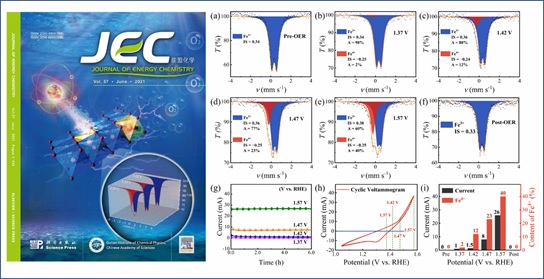
In this work, we used Prussian blue analogues as precursors to prepare Ni-Fe oxyhydroxide with low crystallinity through topological transformation, which has a high OER activity. And it was first discovered that a large amount of Fe4+ (1.42 V vs. RHE, 12%) was formed near the OER starting potential by in-situ/operando electrochemical Mössbauer spectroscopy technology. As the voltage increased, its content can reach 40% (1.57 V vs. RHE). Through systematic research, the research group first confirmed experimentally that the current density of OER is positively correlated with the content of high-valent iron species (Fe4+), and deepened people's understanding of the reaction mechanism of Ni-Fe oxyhydroxide in OER.
Related work was published in full text in the Journal of Energy Chemistry and it was selected as the cover article (https://doi.org/10.1016/j.jechem.2020.09.014). This study is financially supported by the International Partnership Program of Chinese Academy of Sciences (No.121421KYSB20170020).